All About Neurotransmitters
Cholinergic Activity
Given the abundance of long words, we use abbreviations; e.g., the term cholinergic is abbreviated to CH, acetylcholine is usually ACh and, as mentioned previously, anticholinergic burden scale is ACB. There are three types of anticholinergics, which are categorized according to the parts of the nervous system they interfere (block) nerve cell receptivity to ACh. They include; ganglionic blockers, antimuscarinic agents and neuromuscular blockers. Before we discuss the science of blocking (or inhibiting) a neurotransmitter like ACh, we need to understand the neurotransmitter methods we believe serve to transmit information from one neuron to another.
ACh is a neurotransmitter that one neuron sends across a tiny gap, called a synapse, to another neuron's receptor site–which is a protein. ACh is a l molecule that fits or locks into the receptor site; when enough receptors are filled the downstream neuron "fires" and sends neurotransmitters across other synapses, often dozens, to fill receipt sites and trigger further downstream neurons. Neurons look like trees with the receptor site at the top of the trunk (axon), with multiple release points at the tip of its roots (dendrites). Thus a single neuron can send out ACh to dozens of other neuron's receptor sites, which then trigger and send out ACh to thousands more, which once fired send out ACh to millions, etc. all in a split second. The upstream neuron(s) that starts the chain reaction is called "presynaptic" and if enough ACh is released across a synapse and accumulated in downstream, or post-synaptic, neuron it then fires continuing the reaction.
Neurotransmitters are short lived, otherwise a runaway reaction in the nervous system could occur. They are quickly "degraded" or broken down to their constituent parts (like acetyl acid and choline in the case of ACh) by an enzyme called cholinesterase that occurs naturally in the brain. The reason ACh is broken down, ideally after they have crossed the synapse and done their job, is so they can be reabsorbed by spent upstream neurons. The presynaptic neuron regains the constituent parts, where they are reassembled into ACh, and then stored in submicroscopic spheres called vesicules, until the neuron is triggered again to release ACh into the synaptic space. This allows the neuron, once fired, to return to its resting potential–getting ready to fire again and release ACh, which all occurs in microseconds (thousandths of a second). The primary method by which ACh is degraded is by an enzyme called cholinesterase. Enzymes are chemicals that induce a chemical reaction, they don't cause the result–they just help the process. By convention you'll recognize an enzyme as their names typically end with "ase."
Neurotransmitter receptor sites can respond to more than one molecule, as long as they are shaped very similarly. A good model, that has been studied extensively since the 1960's, are opiate receptor sites which are spread throughout the brain, and are also present in the digestive tract and spine. Opiate receptors don't directly trigger the release of pleasure inducing neurotransmitters like dopamine. Instead, they block the production of enzymes that break down dopamine, allowing more of it to be available to activate the pleasure centers. This is similar to how Aricept, Razadyne and Excelon work as they increase ACh bioavailability in the brain by blocking the enzyme (cholinesterase) that degrades ACh, thus indirectly allowing more gas to the brain's engine. Let's digress for a while, and explore the long studied opioid receptor system, as a model for the ACh system, which is also relevant given the increase in opioid addiction occurring in senior citizens around the US.
Dopaminergic Activity
Pleasure centers in the brain are triggered, as everyone knows, can be affected by artificial man-made transmitters like morphine that have a similar shape to the natural opioids that are occur to the human body. These endogenous opioids include; endorphins, enkephalins, dynorhpins, endomorphins and nociceptin. It is thought that this system evolved in humans, and animals, to help mediate complex social behaviors involved in forming stable, "emotionally committed relationships" that facilitated family and social units increasing survival among groups who then passed these genes on. Studies with baby guides pigs found pro-opioid substances (called an agonist) like morphine reduced signs of distress and reduced need/preference to be near their mother, while an opioid antagonist like naltrexone increased distress signs. This has been found in puppies, chicks and young rats. Read more about this fascinating reason we respond to opioids for survival purposes on wikipedia.org.
This is an indirect process, as morphine doesn't directly cause neurons to release more dopamine to activate pleasure centers in the brain. Instead, drugs like heroin and morphine lock into (bind with) opiate receptors which then inhibit the enzymes that break down dopamine.
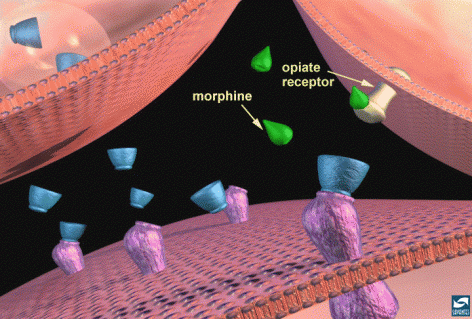
These neurons are located in a part of the brain called the nucleus accumbens. In the depiction above, vesicules (spheres) containing dopamine molecules have reached the end or terminus of the presynaptic neuron (orange left side) and the blue dopamine molecules are being released into the synapse. They cross and bind to the pink opiate receptors which signals the pink neuron to release more dopamine. A theory states that this process is regulated by another neurotransmitter called GABA (Gamma-Aminobutyric Acid), which inhibits dopamine release. Opiate receptors (yellow on the right side) are triggered, it inhibits production of an enzyme (adenylate cyclase) that controls different chemicals that serve to modulate the ability of neurons to fire. If morphine or heroin enters the system and binds to the opiate receptor, this decreases GABA causing less inhibition of dopamine release, allowing the release or more dopamine, increasing the pleasure response.
Addiction/Dependence Model
If drugs like heroin are used frequently, eventually adenylate cyclase adapts to help neurons maintain their normal resting potential and firing rate. Thus, it takes more and more heroin to decrease GABA to get more dopamine produced to gain a "high." This is a major theory behind the tolerance that drug users develop. Unfortunately, tolerance also affects the pain-relieving aspects of morphine, although this involves systems in the brain different from the reward pathway described above. The result is the same, however, it takes more and more morphine to maintain the same level pain relief initially obtained. Eventually, dependence can result when neurons adapt to repeated exposure and start to only function normally in the presence of the substance. If the substance is withdrawn, then withdrawal effects known as a syndrome will occur.
It is important to understand that different brain systems are involved with dependence vs. addiction to drugs like heroin. The above described reward pathways in nerve transmission of dopamine cause addiction, while dependence occurs from neuron adaptation in the thalamus and brainstem areas as a result of compulsive use to get high. Given two systems, one can be dependent on morphine (suffer withdrawal syndrome if it is stopped), but not addicted–this is usually true for terminal cancer patients as they don't use the drug to get high. However, if a person is addicted they are most likely going to be dependent as well. Much of the information in neurotransmitter function comes from the growing addiction science given the increasing compulsive use of opiates to feel good and get high. In fact, information in these sections, including the picture above, was taken from the NIH's National Institute of Drug Abuse publications; e.g., The Neurobiology of Drug Addiction.